Dr. David Martin US Code 15 Explanation of Emergency Use Authorization and Defines “Vaccine”
David Martin, PhD is a well-credentialed entrepreneur in several businesses, an international leader in intellectual property-based financial risk management, patents and has a background in the medical field.
Dr. Martin has received several honors for his contribution to domestic and international entrepreneurial activities. Highlights include:
- Fellow of the Batten Institute at the Darden Graduate School of Business Administration at the University of Virginia;
- Board of Advisors, EU-India, Academy for Augmenting Sustainable Technological Inventions, Innovations, and Traditional Knowledge (AASTIIK);
- Induction as a Guild Member of the Order of King Christian IV of Denmark;
- Recipient of the Charlottesville Venture Group’s Golden Angel Award; and,
- Recipient of the Virginia Piedmont Technology Council’s Spotlight Award.
THINK PEOPLE, THINK!!
Dr. Martin has publications in law, medicine, engineering, finance, and education. He maintains active research in the fields of linguistic genomics, fractal financial-risk modeling, and cellular membrane ionic signaling.
In the videos below, he outlines first, the application of US Code 15 historically upon holistic health practitioners who have been shut down, their materials stolen by the federal government (FTC, FDA, and CDC) and their businesses hogtied to the point of failing and going out of business in some cases. Similar to what is currently happening with the COVID-19 regulations. Key in this statue is the practice of knowingly using terminology with the intention of deceiving the public who are consumers of medical services and products.
He makes an argument that the CDC, WHO, and FDA could potentially be violators of this Federal statute in that both the Pfizer and the Moderna “Vaccines” are Not Vaccines at all, rather they are synthetically created mRNA modifications of the DNA. That said, all the marketing of these products to the public are framing and claiming that they are vaccines which to the lay person means they will a) increase the immunity of the patient toward COVID and b) that it will reduce the spread of the COVID to others. Both a. and b. are specifically not attributes of the mRNA modifications / gene therapies as explicitly stated by these two manufacturers. Additionally, the use of the Emergency Use Authorization (EUA) [ i.e. experimental drug with insufficient testing on animals to be proven via standard FDA processes], combined with the term “vaccine” may actually being utilize to umbrella these two firms under a clause that removes ALL Liability (meaning they cannot be sued) if anything goes wrong with patients.
PATENTS ON CORONA VIRUS
Dr. Martin’s second video explains how the CDC has been registering patents on COVID since 1999 and potentially developing the same earlier. Why then is the CDC the regulatory agency who has authority to waive their own liability as it relates to their products?
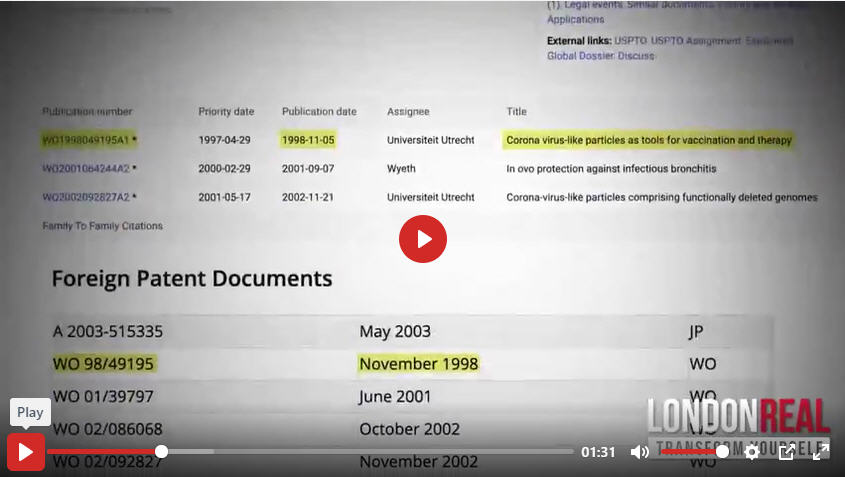
https://patents.google.com/patent/EP1610817B1/en
WO 98/49195 Patent Reference within the above Google Patent entry
Download: WO2002086068A2
Description
[0001]
The present invention is concerned with a vaccine for use in the protection of poultry against infectious bronchitis (IB) comprising an attenuated infectious bronchitis virus (IBV) and a pharmaceutical acceptable carrier or diluent, a method for the preparation of such a vaccine and the use of an attenuated IBV for the manufacture of a vaccine for the protection of poultry against IB for in ovo administration.
[0002]
IBV is a member of the genus Coronavirus, family Coronaviridae. It has a positive sense, single-stranded RNA genome of approximately 28 000 nucleotides associated with a nucleocapsid protein, N, surrounded by a lipid membrane/envelope. Three other viral proteins are associated with the envelope: the large spike glycoprotein, S; a smaller integral membrane protein, M; and the E protein, the smallest of the envelope-associated proteins.
[0003]
The coronavirus S protein is a type I glycoprotein which oligomerises in the endoplasmic reticulum to form trimers which constitute the coronavirus virion spikes observable by electron microscopy. The S protein is assembled into virion membranes, possibly through noncovalent interactions with the M protein, but is not required for formation of coronavirus virus-like particles. Following incorporation into coronavirus particles, determined by the carboxy-terminal domain, the S glycoprotein is responsible for binding to the target cell receptor and fusion of the viral and cellular membranes, fulfilling a major role in the infection of susceptible cells. Furthermore, the IBV spike protein is involved in the induction of a protective immune response when inoculated into chickens (for a review see Cavanagh, in: The Coronaviridae; ed: S.G. Siddell, Plenum Press, 73-113, 1995).
[0004]
All coronavirus S glycoproteins, consist of four domains; a signal sequence, that is cleaved during synthesis, the ectodomain which is present on the outside of the virion particle, the transmembrane region responsible for anchoring the S protein into the lipid bilayer of the virion particle, and the cytoplasmic tail that might interact with other IBV proteins, such as the membrane protein (E) and integral membrane protein (M). The IBV S glycoprotein (1162 amino acids) is cleaved into two subunits, S1 (535 amino acids 90-kDa) and S2 (627 amino acids 84-kDa). The C-terminal S2 subunit associates noncovalently with the N-terminal S1 subunit and contains the transmembrane and C-terminal cytoplasmic tail domains. The S1 subunit contains the receptor-binding activity of the S protein.
[0005]
In previous studies with other coronaviruses, murine hepatitis virus (MHV) and transmissible gastroenteritis virus (TGEV), a spike gene of a (virulent) donor virus strain was used to replace the spike gene of a receiver virus strain to investigate the determinants of pathogenesis and cell tropism. These studies showed that both the in vitro properties (cell tropism) and in vivo properties (virulence) of the donor virus strain were acquired by the receiver virus strain. It was concluded that the spike gene is a determinant of cell tropism and virulence (Phillips et al., J. Virol. 73, 7752- 7760, 1999; Sanchez et al., J. Virol. 73, 7607-7618, 1999; Das Sarma et al., J. Virol. 74, 9206-9213, 2000; Navas et al., J. Virol. 75, 2452-2457, 2001 and Kuo et al., J. Virol. 74, 1393-1406, 2000; international patent application WO 01/39797 ). International patent application WO 98/49195 discloses a coronavirus (e.g MHV) in which a part of the spike protein gene has been replaced by the corresponding part of the spike protein gene of an unrelated coronavirus (e.g. FIPV), thereby acquiring another cell substrate specificity allowing the recombinant virus to target other cell types.
Download: US7279327B2 – Methods for producing recombinant coronavirus
IMPORTANT:
“What is historically important is that Ralph Baric, PhD, professor at the Department of Microbiology & Immunology at the Univ. of North Carolina(Chapel Hill) and Corona virus Patent No. US7279327b2 co-inventor, is reported to have sent a strain of coronavirus to researchers at the Wuhan laboratory in 2015 for “gain of function” studies, as reported in Nature Medicine, Vol. 21, No. 12, pages 1508-13, 2015, given that such studies were banned in the US at the time. Gain of function studies resumed in 2017.”
The above was extracted from the article link here: Download Full Article Here
DO YOUR OWN RESEARCH, DRAW YOUR OWN CONCLUSIONS BASED ON THE ABOVE AND OTHER ARTICLES FREELY AVAILABLE ON THE INTERNET.
FYI, PLEASE USE DUCKDUCKGO.COM AND NOT GOOGLE.COM FOR YOUR SEARCHES. GOOGLE IS PART OF THE DEEP STATE AND IS WATCHING WHAT YOU ARE RESEARCHING AND REMOVING EVIDENCE DAILY.
Note: Something else to think about and call your insurance company to see if consenting to using the Covid Facemasks, the PCR Test or the Vaccine make null and void your current Life Insurance policy. ASK QUESTIONS and get what you have paid for !!! Sadly, this lady in Canada was told her policy was void if she consented to the EUA vaccine.
